Regulatory Affairs
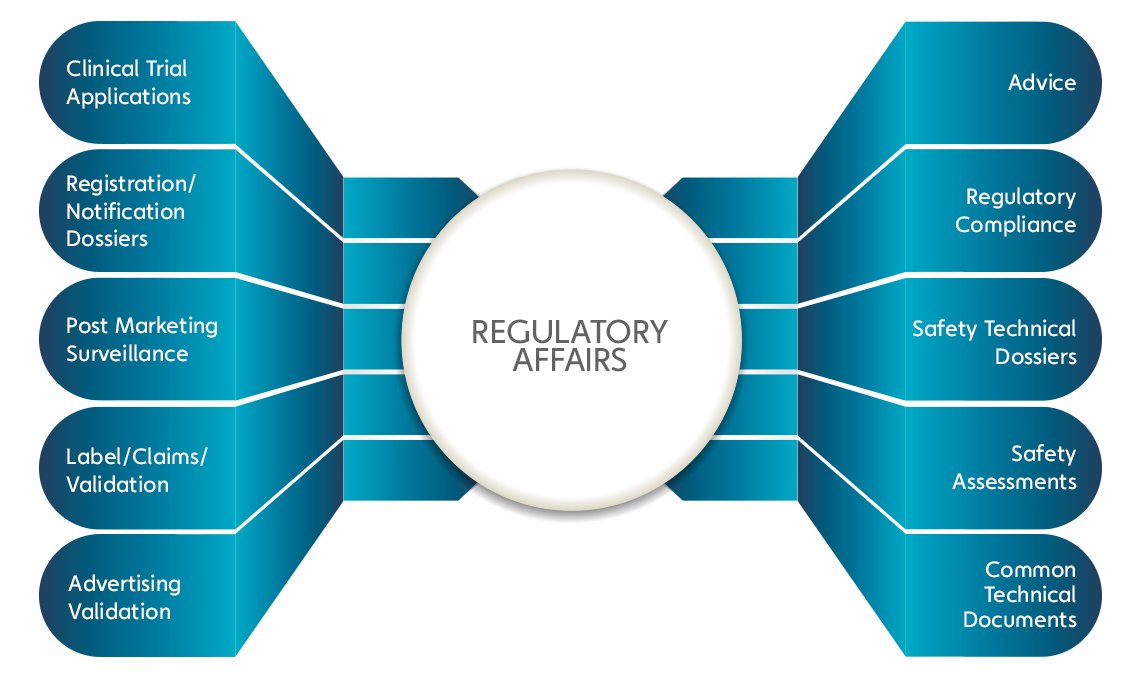
Regulatory Affairs
Navigating the complex landscape of pharmaceutical regulations is crucial for successful global trade. At Zonavista LIfescience, we offer comprehensive regulatory services designed to simplify the compliance process and ensure that your products meet all necessary standards for international markets.
Our team of experts is well-versed in the regulatory requirements of various countries, enabling us to provide tailored guidance for your export needs. From product registration and documentation to quality assurance and adherence to Good Manufacturing Practices (GMP), we are committed to facilitating a seamless experience.
We understand that timely compliance is vital for your business. That’s why we prioritize efficient communication and proactive solutions, ensuring that you stay informed throughout the entire process. Trust us to navigate the regulatory landscape, so you can focus on what you do best—delivering high-quality medicines to those who need them.
Registration Service:
- DMF (CTD format)
- Preparation, Review and Submission
- Dossier Writing and Review
- Dossier Registration
- COA, COPP
- Notarization
Post-Approval Changes:
- Product re-registration and
Renewal of site according to schedule - Post-approval lifecycle maintenance
- Report compilation and publishing
Pre-Registration Service:
- Drug Product DMF
- Development & Preparation of documents
- Content creation and document services

